Name Matrikelnr.
(This was an online exam. The students had access to the internet.)
Problem 1
Problem 2
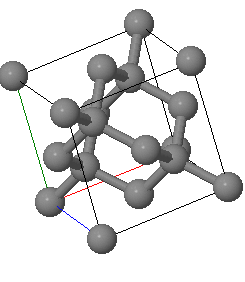
X-rays with an wavelength of 0. Å are used to analyze a crystal with a diamond crystal structure with a lattice constant of Å.

The diffraction peaks are labeled using the conventional (cubic) unit cell. If the primary beam is directed in the positve $x$-direction, what are $\vec{k}$, $\vec{k}'$ and $\vec{G}$ for the reflection?
Problem 3
A metal has an fcc crystal structure with one atom in the basis. The lattice constant is . Å and this metal has one valence electron.
Problem 4
Neutral atoms A and B are far apart from each other. This situation is assigned an energy $E=0$. An electron is taken from atom A and put to atom B. This increases the energy by the ionization energy of atom A, . eV, minus the electron affinity of atom B, 2 eV. The ions are then slowly brought together and as they get closer, the energy of the system decreases like a Coulomb potential. At which distance is the energy zero again?