

All subquestions a, b, c, $\cdots$ are worth 5 points.
Problem 1
  |
Consider a CO2 molecule. A section of the periodic table of atomic masses is shown to the right.
(a) How many rotational and vibrational modes does CO2 have?
(b) CO2 is prepared with 12C and 13C isotopes. The isotope of oxygen is always 16O. How do the rotational and vibrational frequencies compare between the molecules with 12C and the molecules with 13C?
(c) How could we calculate which rotational states are occupied at 300 K?
(d) How many occupied molecular orbitals are there in the ground state of a CO2 molecule? How many are occupied in the first excited state of a CO2 molecule?
Solution
Problem 2
The conventional unit cell of solid CO2 (dry ice) is shown below.
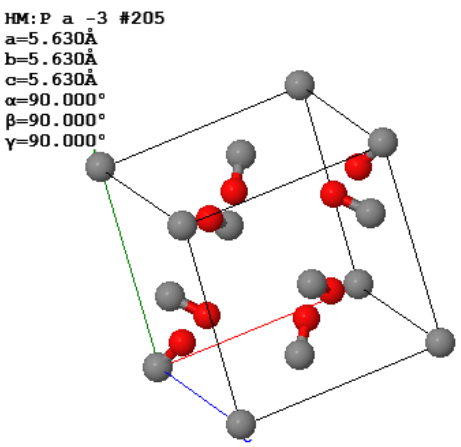 |  |
All of the oxygen atoms (red) are entirely within the conventional unit cell. All of the carbon atoms (gray) are either in the corners or on the faces.
(a) What is the Bravais lattice of this crystal?
(b) What is the smallest value of $2\theta$ in a diffraction experiment of dry ice if the x-rays have a wavelength of 1.54 Å?
(c) How many acoustic branches and how many optical branches are there in the phonon dispersion relation of dry ice?
(d) The electronic band structure of dry ice is shown below. At zero temperature, the states are occupied up to $E=0$.
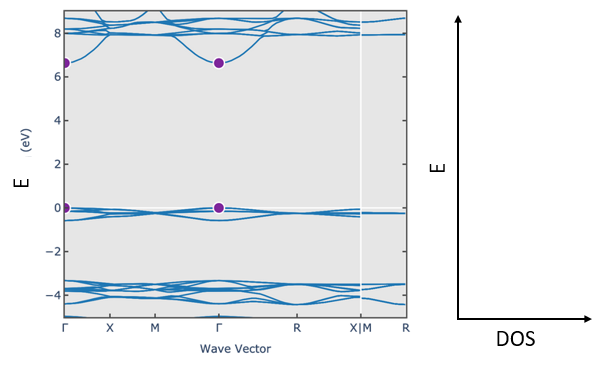
Draw the electron density of states. Is this a metal, a semiconductor, or an insulator? Why? Draw the position of the chemical potential at room temperature.
Solution
Problem 3
Gold has an fcc crystal structure where the lattice constant of the conventional unit cell is, $a=4.079$ Å. Gold is monovalent, and the effective mass of the electrons is $1.26\,m_e$ where $m_e$ is the mass of a free electron.
(a) How could the lattice constant be measured experimentally?
(b) How could the effective mass be measured experimentally and/or calculated theoretically?
(c) What properties of gold could you calculate using this information?
(d) If you wanted to improve your calculations of the properties of gold, what additional information would you need?
Solution