Problem 1
(a) The molecular orbitals are found by solving the molecular orbital Hamiltonian. What is the molecular orbital Hamiltonian for O2?
(b) The molecular orbital Hamiltonian can be solved using the method of Linear Combination of Atomic Orbitals. Suppose we assume that we assume that the molecular orbitals for O2 can be written as a linear combination of the oxygen 1s, 2s, and 2p atomic orbitals. How many molecular orbitals would be calculated?
(c) Write down the ground state many-electron wavefunction for O2.
Problem 2
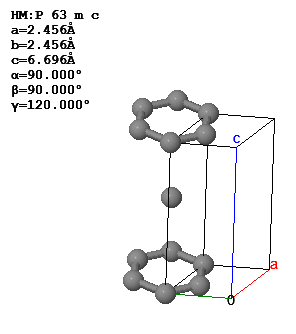
The conventional unit cell and lattice parameters of graphite are shown below.

What is the Bravias lattice, the basis, the primitive lattice vectors, and the volume of the primitive unit cell?
Problem 3
The lattice constants for a hexagonal metal have the relationship $c=2a$. Some distances in the first Brillouin zone are given below.
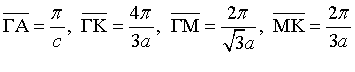
Use the empty lattice approximation to draw the lowest band of the electronic band structure in the directions $A-\Gamma-M-K$.
Sketch the electron density of states near the bottom of the band you have drawn.
Problem 4
By applying stress to a semiconductor, the resistance can be changed. This is called the piezoresistive effect. A change in the arrangement of the atoms in the crystal due to stress will change the band structure.
(a) How can you measure the change in the arrangement of the atoms experimentally?
(b) How will the resistance change if the curvature of the conduction band minimum gets sharper? Why?
(c) How will the resistance change if the band gap of the semiconductor increases? Why?