Useful constants and conversion factors
1 a.m.u. (atomic mass unit) = $1.66 \cdot 10^{-24}$ kg
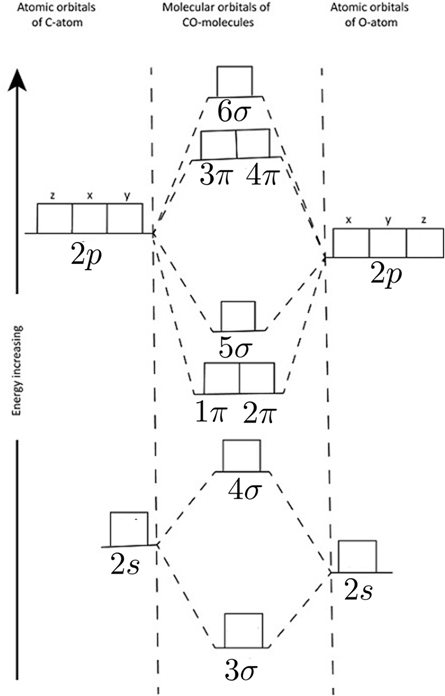
Problem 1 (a) Write down the many-electron Hamiltonian (i.e. the total Hamiltonian) for carbonmonoxide ($Z_C = 6$, $M_C$ = 12 a.m.u., $Z_O = 8$,$M_O$ = 16 a.m.u. ) neglecting Slater's rules (i.e., without accouting for screening or an effective charge $Z_{eff}$. (20% of the points) (b) How many molecular orbitals are filled in the ground state of negatively charged CO (oxygen has 8 protons and 8 neutrons, carbon 6 protons and 6 neutrons) (20% of the points)?
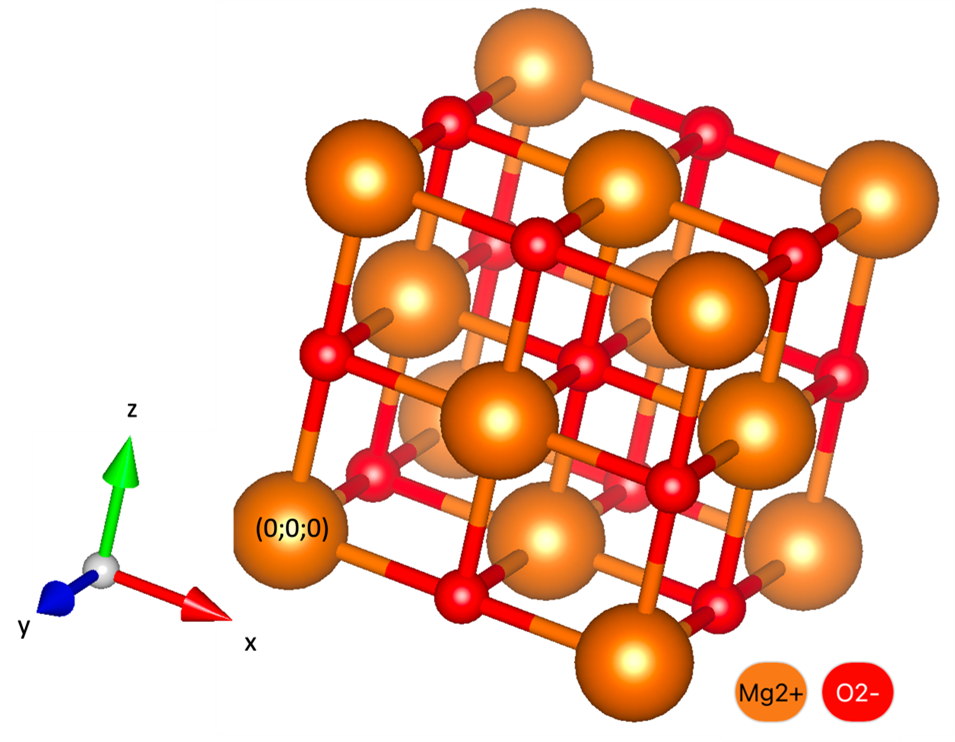
(c) The many electron Hamiltonian is difficult to solve. Write down an approximate many electron wavefunction for the ground state of neutral carbonmonoxide. This wavefunction should be properly antisymmetrized. (20% of the points) (d) How could you determine the bond length of CO from the rotational spectrum? (20% of the points) (e) How could you determine the effective spring constant of an CO2 from the vibrational spectrum? (20% of the points) Solution Problem 2 The potential energy of a diatomic molecule as a function of the interatomic distance $r$ is, The equilibrium interatomic distance was experimentally determined to be 2.5 Å and the binding energy was experimentally determined to be 55 meV. (a) Sketch the bond potential and indicate the two experimentally determined quantities in the drawing. (50% of the points) (b) Show that the equilibrium interatomic distance is $r_e=\left(\frac{2B}{A}\right)^{1/6}$. (20% of the points) (c) Given the model potential above, what is likely to be the dominating bond archetype in this diatomic molecule? (30% of the points) Solution Problem 3 Magnesium Oxide (MgO) is an insulator that crystallize in a rock-salt form (a=4.121 Å). (a) What is the Bravais Lattice? (30% of the total points) (b) How many atoms there are in the basis? What are the positions of the atoms of the basis given in fractional coordinates of the conventional (cubic) unit cell? Please use the reference system indicated in the picture. (30% of the total points) (c) Indicate the (100) plane in the sketch above (please use the reference system indicated in the picture) and draw a top-view of it. (20% of the total points) (d) In the sketch above, mark [001] and [010] directions. (please use the reference system indicated in the picture) (20% of the total points) Solution