Problem 1
(a) Write down the many-electron Hamiltonian (the total Hamiltonian) for carbon ($Z=6$) neglecting Slater's rules.
(b) The many electron Hamiltonian is difficult to solve. Write down an approximate many electron wavefunction for the ground state of carbon. This wavefunction should be properly antisymmetrized.
(c) How could you determine the bond length of O2 from the rotational spectrum?
(d) How could you determine the effective spring constant of an O2 from the vibrational spectrum?
(e) How many molecular orbitals are filled in the ground state of O2? (Oxygen has 8 protons and 8 neutrons).
Solution
Problem 2
(a) Which directions are equivalent to [100] in a tetragonal system?
<100> =
(b) Which planes are equivalent to (110) in an orthorhombic system?
{110} =
(c) A plane cuts the crystalographic axes of a crystal at $3\vec{a}$, $4\vec{b}$, and $6\vec{c}$ where $\vec{a}$, $\vec{b}$, and $\vec{c}$ are the primitive lattice vectors. What are the Miller indices of this plane?
(d) Draw the primitive lattice vectors in this periodic pattern.
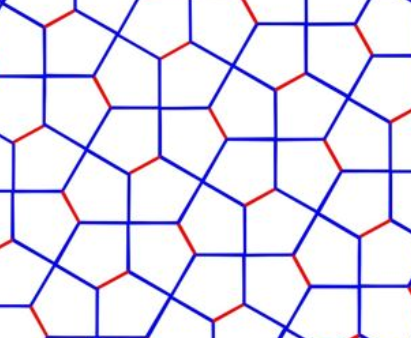
Solution
Problem 3
Two views of the conventional unit cell of hexagonal boron nitride are shown below.
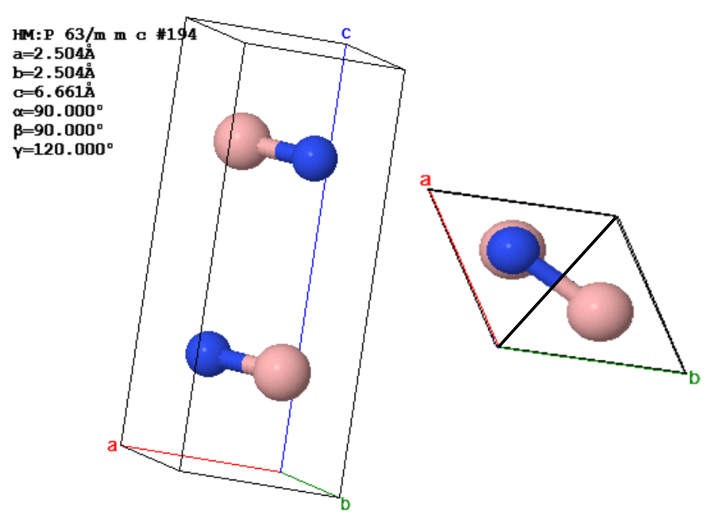
(a) Draw the atoms in the (001) plane.
(b) What is the volume of the conventional unit cell? (Don't forget the units.)
(c) What is the asymmetric unit for this crystal? Explain your reasoning.
(d) What is the space group of this crystal?
(e) The reciprocal lattice vector $\vec{b}_3$ must be perpendicular to $\vec{a}_1$ (= a in the drawing) and perpendicular to $\vec{a}_2$ (= b in the drawing). What is $\vec{b}_3$? (Don't forget the units.)
Solution